Latest News
New Partners at SAXOCON
We are so very pleased to announce the addition of three new partners. Our highly skilled and valued colleagues Carsten B. Senholt, Kirsten Kling, and Martin Hover are now partners at SAXOCON. This happy news is also an important step in our development as a business.
So, three cheers for our three new partners.
Unexpected Findings
Are you responsible for the production of pharmaceutical products such as vaccines? What if you suddenly find something unexpected? What if there is some foreign material or particles in one of the vials in your production?
Sudden unexpected findings of particulate matter in or in contact with drug products are common in pharmaceutical production facilities. Good Manufacturing Practice (GMP) and proper risk management of product quality require identifying the root cause and evaluating the impact of findings on product safety. This knowledge is crucial to deciding whether to release or recall affected product batches.
A quick response is necessary when such a finding occurs. Production must be put on hold while the affected lots are identified, and then the hard work of figuring out the type and extent of the problem begins.
SAXOCON has the in-house expertise and experience to quickly assess, process, and help mitigate any unexpected findings.
Check out how SAXOCON can help you here.
3D Printing and Your Health
Are you using 3D printers to produce mock-ups or in manufacturing? What kind and how many particles are produced while using a 3D printer? Particles produced during 3D printing pose a health risk. In fact, they are almost as harmful as the exhaust produced by Diesel motors. Ensuring good air quality and the health and safety of your workplace requires knowing what is in the air you are breathing.
Current rules and regulations for determining air quality use particle mass for determining what level of pollution is acceptable. In fact, the toxicity of a particle is determined by a variety of attributes other than mass. Additionally, the number of particles in a given space plays a role in your exposure. This determines how many particles end up inside you and what the consequences are for your health.
SAXOCON can assess your workspace design on-site, identify sources and measure particle concentrations.
Check out how we can help here.
Food Contact Materials
Packaging is not the first thing we think of when we think of food. However, the materials used in food packaging impact the food contained within. So, it is essential to understand the health and safety consequences presented by the food packaging used.
Any materials used for food packaging, known as food contact materials, must be evaluated for their potential for transferring harmful substances to food. Every supplier in the supply chain for food contact materials is responsible for demonstrating compliance with all applicable rules and regulations regarding food contact materials.
SAXOCON can help you ensure that your materials comply with EU and FDA rules concerning food contact materials.
Check out how we can help here.
Happy Easter! ![]()
![]()
Get Ready for EU MDR
The deadline, 26 May 2021, for EU MDR is fast approaching.
The Danish Medicines Agency (Lægemiddelstyrelsen) recently published guidance regarding the new regulations and their consequences for both industry and Danish authorities. Read their guidance here (Danish only).
Keep abreast of the latest news and information regarding MDR and make sure your products are ready for the new regulations. SAXOCON can help you to ensure your products are qualified.
Are you ready? We are.
Check out how we can help here.
Material Readiness
Whether you are manufacturing medical devices, pharmaceuticals or combination products, you will be using materials. Marketing your products in the EU requires that you ensure the quality and safety of the materials used in your products. Early and robust material selection is key to meeting regulatory requirements and mitigating the risk of unexpected events.
In our latest webinar, SAXOCON CTO, Carsten B. Senholt, discusses the importance of materials readiness and its role in the proper selection of robust materials candidates for production.
Surface Characterisation
Surfaces are everywhere, and we constantly come into contact with them. To live in this world means touching surfaces, so we rarely give it any thought. But the things that we manufacture that we come into contact with must be safe. This is true for everything we use or wear but especially so for medical devices.
Any medical device that comes into direct contact with people’s skin, blood or organs; or has indirect contact via contact with food or by conducting air or fluids that interact with people must be CE Certified to be marketed in the EU. Depending on their duration and type of body contact must undergo a physicochemical, morphological, and topographical (PMT) analysis, as defined by ISO 10993-19.
Correctly conducting a PMT analysis requires care and skill and is a critical step in ensuring product safety. Once completed, you will be better prepared to make well-informed decisions about your medical device and its readiness for the market.
SAXOCON has the expertise to help you get your medical devices compliant and certified for sale in the EU.
Check out how we can help here.
SAXOCON CTO at the Extractables and Leachables Forum 2021
SAXOCON is pleased to announce that our CTO, Carsten B. Senholt, will present at the 2021 Extratables and Leachables forum.
Carsten will discuss the importance of materials readiness and its role in the proper selection of robust materials candidates for production.
Proper materials selection is key to an intelligent and cost-effective extractables and leachables test strategy.
More information about the forum can be found here.
Safe Stents and Implants
Stents and other medical implants are products that have a high level of contact with the human body. As such, they are subject to strict regulations regarding their safety. If you are a manufacturer of stents or implants and want to market them in the EU, you will need to apply for a CE Mark certificate. This certificate verifies that the product is safe and performs as intended. The ISO 10993-1 standard prescribes a process for evaluating and testing the biological safety of a medical device product, including the physicochemical, morphological, and topographical characterization of materials.
SAXOCON has the necessary expertise to help you get your stents and implants compliant and certified for sale in the EU.
Check out how we can help here.
Indoor Air Quality Investigations
Air quality problems can often be difficult to pinpoint? You can be doing everything by the book concerning air temperature, humidity, and CO2 levels and still have an air-quality problem. In most cases, when confronted with persistent and difficult-to-diagnose, air-quality mysteries, the culprit is Ultra Fine Particles (UFP).
UFPs are defined as those particles with a diameter of less than 0.1 micrometres. Such particles make up the vast majority of particles found but only account for a fraction of the present mass.
UFPs are usually the by-products of combustion or chemical reactions, such as:
- Laser printers
- Cleaning agents and chemical storage
- Kitchen usage and smoking areas
- Boiler and furnace leaks
- HVAC duct and filter leaks
- Grinding, welding, soldering, and powder handling
- Vehicle emissions and outdoor air pollutants sources
Due to their small size, UFPs often travel along unexpected pathways to areas that may be far from their source.
SAXOCON can help you find the emitting source(s) and to control or even remove them.
Check out how we can help you here.
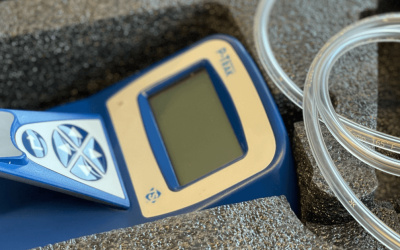
Secure Worker Health and Safety
Do you know what particles your workers are exposed to in your working environment? Which of these particles is a concern for the health of your workers? Usually, understanding the risks posed by particles associated with processes that generate dust or involve handling powder requires long-term comprehensive studies to assess potential toxicological exposure.
Our novel tool, the SAXOCON Impactor, quickly collects particles from the air in your workplace directly onto electron microscopy grids, which allows us to accelerate the assessment process.
Check out how we can help here.
Safe Reusable Medical Devices
Reusable medical devices are ubiquitous and necessary tools for medical facilities around the world. As a manufacturer of reusable medical devices who wants to sell them in the EU, you will need to ensure that your cleaning, disinfection, and sterilisation procedures are validated.
SAXOCON has the expertise to help you get into compliance with all relevant guidelines, such as AAMI TIR12 and ISO 17665, and keep your products on the market or get your new products approved in a timely manner.